Body’s own immune system identified as a potential culprit in causing cavities, damaging fillings
U of T study provides first evidence that bacteria are not solely responsible for tooth decay
A new study by researchers at the University of Toronto’s Faculty of Dentistry provides the first evidence that the body’s own defence system could be a major contributor to tooth decay and filling failure.
Published in biomedical journal Acta Biomaterialia last week, the study shows that the decay of dentin (that hard substance beneath the tooth’s enamel) and fillings is caused not just by bacteria alone — as has been the model understood by science for decades — but through the unique activity of oral immune cells known as neutrophils, which potentially enhance the effects of bacteria.
“No one would believe that our immune system would play a part in creating cavities,” says lead author of the study, Yoav Finer, who holds the George Zarb/Nobel Biocare chair in prosthodontics at the Faculty of Dentistry. “Now we have evidence.”
Recognized as important players in combatting inflammation in all parts of the body, neutrophils are a type of short-lived innate immune system cell that enter the oral cavity from the gums around the roots of the teeth.
"No one would believe that our immune system would play a part in creating cavities."
When bacteria invade the oral cavity, the body sends out neutrophils to attack. But as the neutrophils track down and destroy the invading bacteria, they may cause destruction to the localized environment as well as to the bacteria.
“It’s like when you take a sledgehammer to hit a fly on the wall,” Finer explains. “That’s what happens when neutrophils fight invaders.”
It’s not just the force of the neutrophil invasion, however, but the by-products of the attacks that cause the damage.
On their own, neutrophils are incapable of causing damage to the teeth, explains Finer: “They don’t have acid, so they can’t do much to mineralized tooth structures.”
But when neutrophils engage in attack, the acids produced by the oral bacteria demineralize the tooth. That’s when enzymes from both the immune cells as well as their targets rapaciously eat through teeth, and can also cause collateral damage to tooth-coloured fillings.
And it’s a speedy process: “Within hours,” the researchers found, dentin and tooth-coloured fillings sustain damage.
“It’s a collaboration of destruction — with different motives,” says study author Michael Glogauer, professor at the Faculty of Dentistry and acting chief dentist at the Princess Margaret Cancer Centre.
Corroborating previous studies conducted by the group, the research could also explain why so many patients whose caries are treated with tooth-coloured fillings face high rates of recurrence of the disease. Most tooth-coloured fillings fail within five-to-seven years, costing Canadians approximately $3 billion a year.
"Ours is the first basic study to show that neutrophils can break down resin composites (tooth-coloured fillings) and demineralize tooth dentin,” says M.Sc. student and first author of the paper, Russel Gitalis. “This suggests that neutrophils could contribute to tooth decay and recurrent caries.” Gitalis, who was previously awarded a research scholarship from the Canadian Institutes of Health Research to pursue the research, hopes the study will lead to new assessments for caries risk and treatment strategies.

Implications for novel therapeutics
While the study provides the first direct evidence that an immune response may contribute to tooth decay, it also opens up new avenues for research.
“We can develop new methods to prevent immune-mediated destruction of teeth,” says Glogauer.
The findings may also one day lead to new standards for testing filling materials, says Finer, who argues that materials need to face trial within the collaborative destruction lab to pass muster. “We need to test interactions with the body and bacteria,” he adds.
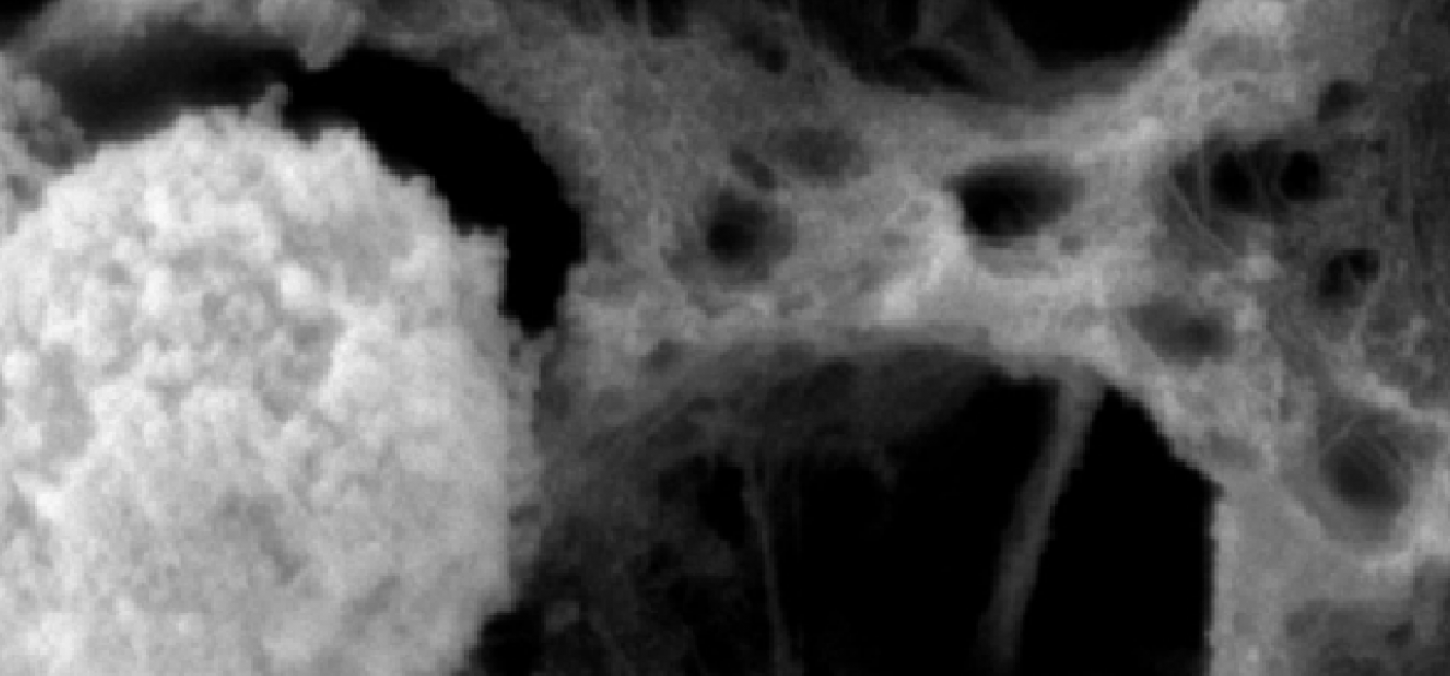
Top image: Collagen after incubation with neutrophils for 24 hours: image depicts a loss of collagen fibril network when incubated with neutrophils. Image courtesy Russel Gitalis
Above: Yoav Finer, courtesy Jeff Comber