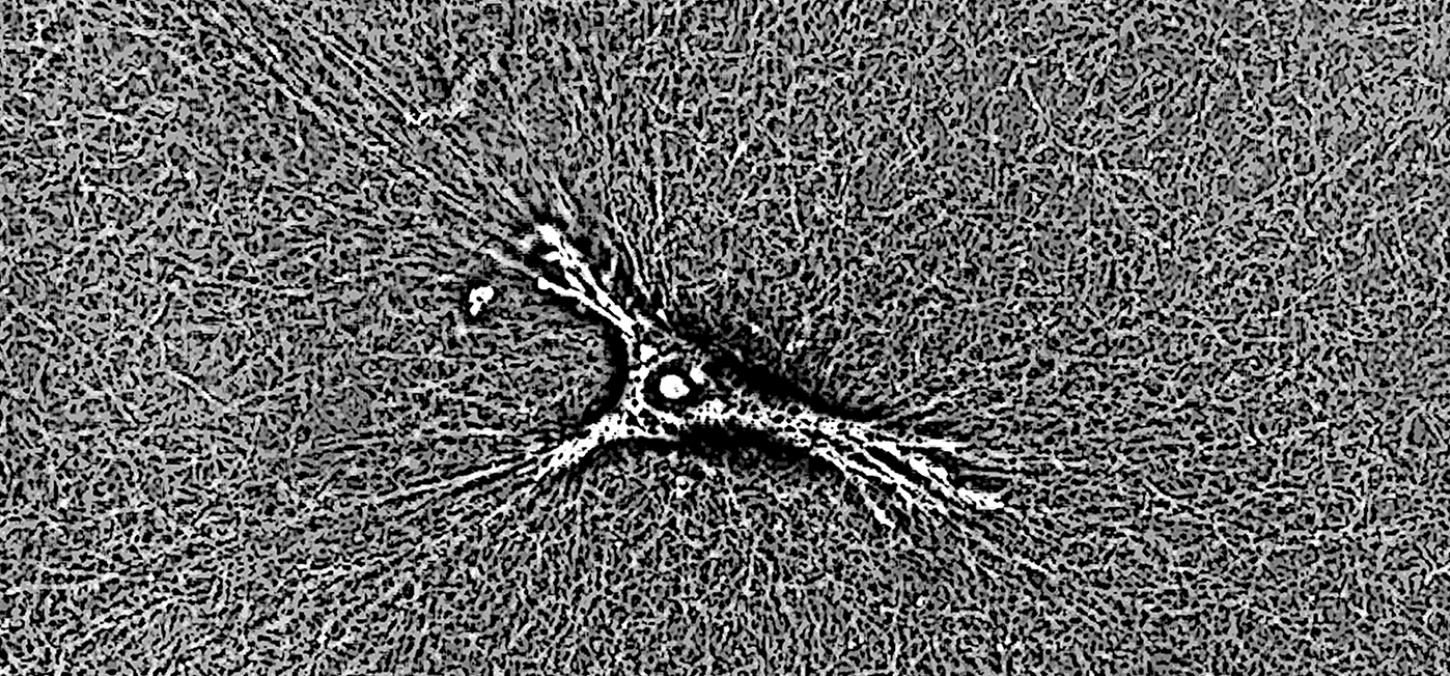
Fibroblasts communicate mechanically over lengthy distances: study
It’s been likened to a spider pulling at the threads of its web. But instead of silk and spider legs, this describes the recently-discovered mechanical communication process between macrophages and fibroblasts in the extra cellular matrix.
Published last month in Nature Communications, researchers at the University of Toronto’s Faculty of Dentistry have uncovered a previously unknown communication strategy between the body’s repair cells, fibroblasts and macrophages, which may one day reveal new understandings about wound repair and fibrosis.
When an area of the body is injured, fibroblast cells are called in to remodel the extra cellular matrix, which in turn repairs the tissue.
Previous research concluded that the communication between these cells were mainly mediated by chemical signals. Cellular chemical signals are relayed over short distances — to the length of a half millimetre between cells.
Using live imaging microscopy, the researchers were surprised to find a second strategy at play, as the macrophages are able to perceive fibroblast cells (which are themselves only one tenth of a millimetre in diameter) more than one millimetre away.
The theory is that, as the fibroblasts pull and tug on the fibres of the extracellular matrix, the spider-like macrophages are able to feel the vibrations across the fibres. They are able to hone-in on the origins of the mechanical signal and move rapidly towards it.
Bodies have evolved in a way can have a second communication strategy between the cells for when the chemical environment is too complex"
Supporting their claim, the researchers found that the mechanical ‘homing device’ strategy of the macrophages worked both with the fibroblasts, and with a placebo bait. Using a microneedle, the researchers were able to conclude that similar tugging movements on the extra cellular matrix — rather than chemical signals — caused the macrophages to move in.
“Bodies have evolved in a way can have a second communication strategy between the cells for when the chemical environment is too complex,” says the study’s first author, postdoctoral fellow Pardis Pakshir. “It’s not a reserve strategy. The cells will respond to chemistry and mechanics simultaneously.”
Whether it works to the body’s advantage, however, is another story. “This could be good —a signal for the macrophages to target cells, or to support them in another way or change their behaviour. Or it could be a disease mechanism,” says Boris Hinz, distinguished university professor at the University of Toronto’s Faculty of Dentistry and corresponding author of the study. “We just don’t know yet.”
The fundamental discovery could help researchers gain greater understanding on the mechanics behind fibrosis, where an overactive wound healing response leads to scarring, and even organ and tissue death.
Image: extracellular matrix, courtesy Pardis Pakshir