
Harnessing a naturally occurring process for tissue engineering applications
By Nina Ambros
U of T Dentistry’s, Mina Vaez’s research article titled “Modulation of the biophysical and biochemical properties of collagen by glycation for tissue engineering applications” was recently published in Elsevier’s Acta Biomaterialia journal, a highly respected publication that focuses on the field of biomaterials science.
Originally from Iran, Mina studied at the University of Tehran before moving to Canada to pursue her PhD in biomaterials and biomedical engineering. She is currently in her third year of the program and is supervised by Dr. Laurent Bozec.
Changing the bad rep of glycation
In scientific literature, glycation is often associated with pathological conditions such as diabetes. Glycation has a negative connotation and is understood as a mechanism that impairs normal cellular and tissue function.
Mina’s research shows a new side of glycation. It shows that glycation can be harnessed as a potential mechanism to cross-link collagen scaffolds and has important applications for tissue engineering.
Tissue engineering is an evolving field that involves repairing or replacing body tissues in a lab environment, that can then be used to treat patients. “For example, to better treat patients who have lost a large area of skin because of an accident or a burn, scientists are trying to produce skin substitutes in the lab to reduce the need for taking a skin graft from another part of the patient’s body.” says Vaez.
Collagen’s role in tissue engineering
Collagen is the most abundant protein in the body, it stabilizes connective tissue. Collagen scaffolds are a porous network of collagen through which cells can permeate and perform their regenerative functions. Collagen scaffolds are a great option for tissue engineering applications.
Currently, when tissue engineers want to build collagen scaffolds in a lab, the scaffolds can have poor mechanical stability. Cross-linking is a process that stabilizes these structures. However, the cross-linkers that are used widely in the sector can be toxic, or too weak to hold the desired structure together.
The human body has two main cross-linking pathways, a non-enzymatic pathway and an enzymatic pathway. Mina is studying both during her PhD. In her recent research article, she explores the non-enzymatic pathway of glycation. Mina looked at the interaction between collagen and methylglyoxal (MGO) that produced Advanced Glycation End-products (AGEs). Accumulation of AGEs produces intermolecular crosslinks.
“There is a need for a better solution to stabilize the collagen scaffolds. That’s why I decided to explore trying to replicate what is naturally occurring in the human body in my research. AGEs are naturally produced in the body as part of an aging process.” says Vaez.
Modifying properties of collagen
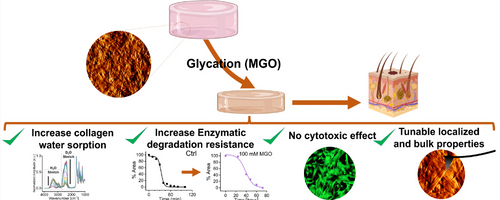
Mina’s research showed unique variations in the properties of collagen following the accumulation of AGEs. She found that accumulation of AGEs increased the stiffness of collagen scaffolds.
“Having the ability to modify the stiffness of collagen is important because it would help personalize tissue engineering to replicate the exact mechanical strength of a patient’s tissue.” says Vaez.
Another finding was that accumulation of AGEs affected the proteolytic degradation rate of collagen. Tissue engineering can come with the risk of immune rejection, when the body of a patient does not accept the new tissue. “It’s crucial that a lab-engineered collagen scaffold can match the patient’s body enzymatic degradation rate.”
Accumulation of AGEs also affected water absorption of collagen. When a collagen scaffold is being made in a lab environment, one of the important factors is its capacity to absorb water. In the human body, interstitial water is responsible for maintaining the mechanical stability of a scaffold.
Applying fundamental science to clinical practice
Mina’s fundamental science research has tremendous clinical applications. Her research can be applied to dentistry, through the glycation of collagen-based membranes for guided bone regeneration.
Tissue engineering continues to evolve and push boundaries for restoring skin, tendons, cartilage, heart and bone. “I hope that my findings serve as a foundation for even more clinical applications that can help patients and make an impact” says Vaez.