
Fibrosis may become a real concern for patients recovering from COVID-19: Meet the U of T researcher who may one day discover how to fix it
As the first wave of COVID-19 infections begin to abate, physicians are discovering that acute cases are developing damage to their lungs and even hearts.
Known as fibrosis, this organ damage could potentially lead to many more problems for these patients down the road.
But what is fibrosis exactly? And is it something we need to worry about? Distinguished professor Boris Hinz, an expert in fibrosis research at the Faculty of Dentistry, cross appointed at the Institute of Biomaterials and Biomedical Engineering, and co-director of the Fibrosis Research Network, explains the ins and outs of this often deadly disorder —and how patients with COVID-19 could one day help researchers find new and better treatments.
What is fibrosis?
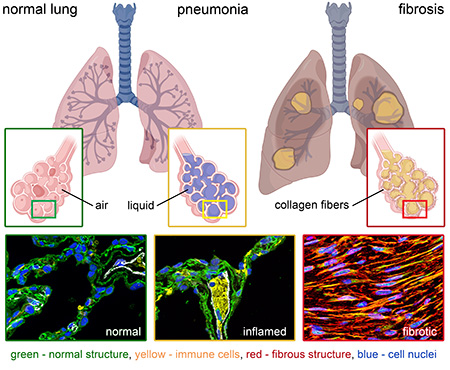
It’s difficult for me to admit but we don’t know what it is exactly, because fibrosis is a group of complex and diverse conditions. But you can think of fibrosis as a scarring of organs. When the body or an organ is injured, repair cells comes in to fill in the wound with collagen — you can think of it as a kind of glue. In fact, “collagen” translates from the Greek word “kólla,” which means “glue.”
When this wound repair mechanism is working properly, it has a protective and integrative function for that tissue. Fibrosis sets in when organs are so severely or repeatedly damaged that they will not regenerate. There will be a blob of stiff collagen where once there were functional cells. If there’s too much of that glue the flexibility of that organ, necessary to its function, is lost. For instance, a fibrotic heart cannot contract and relax, and fibrotic lungs cannot fully expand during breathing.
"If we can find out what the really early stages of fibrosis are through this COVID-19 infection cohort maybe we can learn to intervene earlier."
If part of the organ is not functional anymore, or if there becomes more collagen than functional parts, that impacts negatively on the organs and can lead to death.
One problem is that all of the fibrotic changes that occur in these organs can occur over a very long scale — months, years, even decades. Typically, fibrosis is not visible to any standard diagnosis, so we wouldn’t see those alterations in a regular checkup. By the time someone presents in the hospital with symptoms like shortness of breath, the fibrosis is so progressed, and the damage so bad, that we can’t stage successful interventions.
COVID-19 seems to be creating fibrosis-like conditions in organs such as lungs. What is happening?
With COVID-19, the virus first attacks the epithelial cells of the lungs. When these cells meet the virus, they cry for help by sending chemical signals to nearby blood vessels that contain our immune cells. The body then quickly reacts to the attack of that virus and raises an immune response.
If the acute respiratory infection is very high, like in COVID-19, blood vessels try to get out as many inflammatory cells and anti-viral agents as possible. This causes the blood vessels to become leaky, and they leak fluid into your lung space, which fill up slowly with liquid. They’re not supposed to be. They are supposed to be exchanging air, so there are severe and instantaneous (acute) breathing problems (respiratory stress). Severe Acute Respiratory Syndrome is in the name of this virus class: SARS-cov-2.
So, what’s that to do with fibrosis? If inflammation and water in the lungs persist, frustrated immune cells and damaged epithelial cells now both cry for help from tissue repair cells: fibroblasts. These cells make the collagen that replaces damaged lung structures. In that case, the organ doesn’t regenerate. The body is just using a quick fix to survive, like super glue on something that is damaged. At that point you have fibrosis, scarring of the lungs.
You can think of this lung repair response in the same time scale as a scar forming after you cut finger. Although we don’t really know yet, with acute and strong viral lung infections like those COVID-19, those scars typically don’t progress. They don’t get any better, but they also don’t get worse. It will usually take a year or more for the scars to mature. Typically, they will not resolve, so that impairs some function depending on how badly you’re scarred. Later on, it will affect how you breathe.
What we can hope for with COVID-19 is that the damage or scarring may not progress, like what we’ve seen from previous virus pandemics like SARS. In contrast, with other fibrotic diseases, like idiopathic lung fibrosis, scarring progresses over time.
Could fibrosis research benefit from studying COVID-19 survivors?
My goal as a researcher is to stop fibrosis, and I do that by looking at the fundamental mechanisms underlying how fibrosis develops, and how eventually it can be treated.
Currently, there’s just no cure. In fact, there are only two drugs approved to treat lung fibrosis, but those drugs merely reduce the speed of progression. I’d like to help develop better pharmaceuticals to halt fibrotic progression at the point of diagnosis. That may sound simple, but it’s not. As I mentioned, typically by the time a patient is diagnosed it’s usually quite late. If we could discover the markers for early onset fibrosis, maybe early intervention can save lives.
That’s where patients recovering from acute COVID-19 infections can help us: for these individuals, we know when the damage happened. In time, there will be a lot of follow up with patients who were in ICU. Fundamental researchers like me need to keep collaborating with clinicians in following up with these patients, because fibrosis will develop as consequence of acute or chronic injury.
If we can find out what the really early stages of fibrosis are through this COVID-19 infection cohort — or why certain cohorts develop fibrosis and why others won’t, whether there are underlying genetic reasons for it, or mechanical reasons — maybe we can learn to intervene earlier.
This kind of research might even help us to deal with acute organ injuries: if we can create drug interventions which tone down or better control the initial healing response, that might reduce the secondary- or long-term effects of fibrosis.
What we scientists are struggling with, though, is that fibrosis is initially not a bad thing. We need to keep beneficial repair going and take away the aggressive part of that mechanism. There’s a balance there that we have to get right.
Read more on Hinz's latest fibrosis discovery, the "infinite hug".
Photos: top: inflamed lung tissue (Boris Hinz)