
U of T Researchers Map Regenerative Cells in Implant Wounds
By Diane Peters
A new study out of the Faculty of Dentistry takes a close look at the healing process that happens around implants at the cellular level for the first time.
First author Niloufar Khosravi, PhD [2019] conducted this study using a multiphoton microscope to observe components of tissue regeneration around metallic implants with both smooth and rough surfaces.
“We were able to look at healing at the implant site while it’s happening,” says Khosravi. “The resolution is down to a single cell level, which means you can see the cells while they’re alive and moving. This approach is quite novel in the field of implant dentistry.”
Khosravi worked on the study with Faculty of Dentistry and Institute of Biomedical Engineering professor John Davies and Ralph DaCosta from the Faculty of Medical Biophysics and University Health Network as part of her PhD in Dentistry.
For the study, which is published in Biomaterials, she was able to observe progenitor cells, whose job it is to heal tissues, working to heal the wound and trigger the creation of new blood vessels.
The rough implants experienced what Davies dubbed a “proliferative bloom” of progenitor cells. “What was surprising was not only the number of these cells changing with the surface properties of the implant — the rougher implant had a far greater number of these essential cells. But they also arrived at the wound site earlier,” says Davies.
Smooth implants also caused the creation of these cells, but they came later and in lower volumes, without the dramatic bloom of their rough counterparts.
“When blood vessels start growing in a wound, at some point they have to stop, mature and start remodeling,” says Khosravi. “You don’t want overgrowth of blood vessels and ongoing inflammation.”
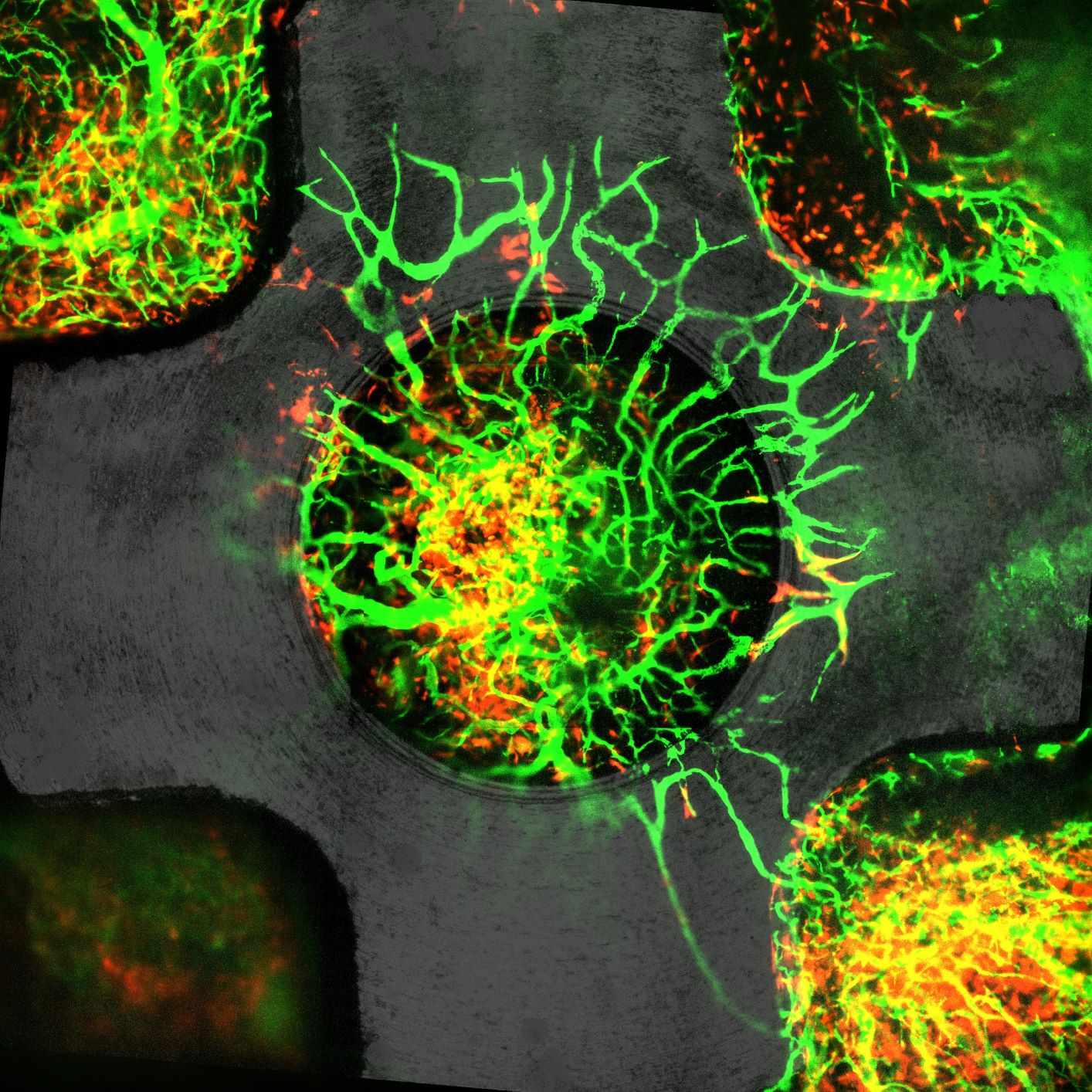
Rough implants and their proliferative bloom had this important ability to switch off at the right time and pivot to a more mature, calmer state to allow for full wound healing without any overgrowth of blood vessels or continued inflammation.
Since this study has tracked many key wound-healing cellular processes, it offers many possibilities for future research.
Davies says this cellular change from generating new blood vessels to a more mature healing state is still a mystery and merits more investigation. “Very little is known about this switch.”
Khosravi notes that this study was done in healthy conditions. Understanding how the healing process changes for some disease states could improve care of patients in future. “From a clinical standpoint, you could personalize the approach for different patients with different healing conditions. Once you know enough about the condition of the cells involved in healing, you could understand why an implant is failing in some patients.”
Photo: Niloufar Khosravi (Jeff Comber)